Cell Biology
Cytoskeleton
We now take a look at a dynamic network of structural and associated
proteins that play important roles in maintaining cell integrity and in
generating cell movements.
Structure and
Function
Cell shapes and sub-cell shapes are directly influenced by the cytoskeleton.
Amoeba pseudopodia, epithelial microvilli in the small intestine, and biconcave
erythrocytes are just a few examples.
Movement by such proteins are evident in striated muscle contraction,
mitosis, amoeba, flagella, and cilia.
Cytoskeletal
Elements
Cytoskeletal
Elements (CSE) include:
microtubules (24 nm diameter)
intermediate filaments (10
nm diameter)
microfilaments (7 nm diameter)
Common Themes of Cytoskeletal Elements
1) soluble, globular protein subunits make up insoluble, linear fibrous
polymers by covalent assembly
2) rapid exchange between a subunit pool of quaternary globular proteins
and a polymer (self-assembly process) "dynamic
instability" that is not in equilibrium.
3) association of subunits involved in non-covalent interactions
4) microtubules (MT) and microfilaments (MF) the form filaments that
are helical with periodically placed interaction sites. This provides
multiple equivalent binding sites.
5) MT and MF form filaments that are polar and all CSEs have readily
distinguishable ends. (The cell knows which way it needs to be moved
in relation to (+) and (-) ends.
6) Demonstrate hierarchy of interactions (protein-protein interactions)
7) are evolutionary conserved - however they often exist as multiple
gene families
8) can be LABILE (sensitive to disassembly) and permanent (or semi-permanent)
9) functioning of CSE involves ASSOCIATION WITH OTHER PROTEINS
-microtubule-associated
proteins (MAPs)
-actin-associated proteins
(actin binding proteins)
-intermediate filament associated
proteins (IFAPs)
Mediation by
Associated Proteins
Types of processes mediated by associated proteins include:
1) affect assembly, disassembly, and stability
- bind to monomers, prevent assembly
- initiate assembly
- promote assembly
- cause disassembly
- subunit modification
- length determination
2) mediate interactions
- cross-link filaments by forming bundles or networks
- link filaments to the cellular structure (including membranes)
- bind along side to strenghen filaments
3) generate movements by mechanoenzymes called "motor proteins"
- convert chemical potential energy of ATP to mechanical energy
Microtubules
and Their Properties
Microtubules (MT) are made up of various subunits are different levels...
Polymers of tubulin
are made of alpha and beta tubulin. Each are 55,000 daltons.
At the quaternary structure are alpha-beta tubulin dimers (110,000 daltons,
8 nm long).
Non-covalent interactions between dimers create PROTOFILAMENTS
Protofilaments associate side to side to form MICROTUBULES
-----
These microtubules have dynamic properties. They are reversible
by re/depolymerization.
High temperatures = association of MTs
Low temperatures = dissociation of MTs
In a test tube, a MT's length over time will go through lag, log, and
stationary phases. The lag phase is nucleation of a sheet to form
a ring. Log is the exponential growth that quickly arises after the
initial nucleation centers have been created. The presence of native
or the addition of MT pieces will speed up the process by overcoming the
lag.
A unique "treadmilling" property is found in a MT where the (+) end
is the addition end with a "net assembly." The (-) end has a "net
disassembly."
Antimicrotubule drugs have been used in the fight against cancer by
interfering with mitotic spindle fibers.
---
colchicine,
colcemid, nocodazole
inhibits addition of tubulin to MT, increases polyploidy
vinblastine, vinoristine (from Madagascar periwinkle)
aggregate the tubulin dimers (used against testicular cancer)
taxol
(from the bark of ewe trees)
stabilizes MTs by binding to them; the cell is unable to get rid of
MTs and mitosis goes haywire (1st drug with major success
against ovarian cancer)
In the cell context, MT don't exist with both + and - ends free.
The microtubule organizing center (MTOC) lengthens and shortens the
+ end only. In cells, the minus end of a MT is usually embedded in
the MTOC and is not a site of tubulin assembly.
The MTOC is the major location of a 3rd tubulin. This isotype
is known as gamma tubulin. Gamma tubulin serves to nucleate MTs.
How do MTs lengthen ans shorten at the same end (known as dynamic instability)?
GTP, yes GTP, is required for MT assembly, and GTP
hydrolysis accompanies this but with a slight dealy. GDP bound
tubulin has a higher rate of disassembly while GTP bound tubulin has a
higher rate of assembly.
A growing MT has a GTP
cap (single ring on + end) on GTP tubulin. If hydrolysis catches
up with assembly, a GDP tubulin cap is present, and MT rapidly shortens.
Microfilaments
and Intermediate filaments
Let's look at the actin-subunits of actin microfilaments.
Globular (G) actin make up filamentous actin (F-actin
microfilaments) polymers (45,000 daltons).
Intermediate filaments (IF) have fibrous, hydrophobic cores with globular
ends that give variation. They can be found as dimers and tetramers.
Cytoskeletal
Mediated Phenomenons
Motor proteins help maintain cell shape via MT and actin based motors.
Cell/Organelle Shape
1)
Erythrocyte membrane skeleton - the behavior of the network of actin
is understood and used to predict how blood cell shapes come about in hypotonic
and hypertonic solutions. These anucleated cells maintain their biconcave
discs despite the harsh conditions of being squeezed through capillaries.
They do this by storing energy in its erythrocyte membrane skeleton (like
a spring, as Melanie mentioned in class). Proteins involved in the
plasma membrane of an erythrocyte include:
Band 3 - a carbohydrate-containing, membrane-spanning
protein present as a dimer with 2 identical subunits which span the membrane
at least a dozen times each. It allows for passive exchange of anions
across the membrane. Its name comes from its position in an electrophorectic
gel.
Glycophoryin A - a carbohydrate-containing, membrane-spanning
protein that spans the membrane once and contains 16 oligosaccharide chains.
Ankyrin, Spectrin, Actin, and Tropomyosins are other
noteworthy proteins.
2) Dystrophin
-
binds to actin and dystrophin associated protein (in the membrane) to the
extracellular matrix. It has been involved with an inherited, x-link
recessive disorder (part of X chromosome is missing in patients with multiple
problems), but why does it have a relatively large number of occurences
in people with no history of this disorder? It is the largest human
gene so it will statistically have a larger chance of mutation than other
genes. The problem stems from a weakness where dystrophin is not
connected to the cell.
3) Microvilli - an end cap
maintains its shape
4) Nuclear lamin - a network
that makes up the nuclear lamina and supports the nuclear envelope.
How does it keep its shape?
It is the target of a cyclin dependent kinase. Phosphorylation
will cause its disassembly. Human mitosis requires break down of
the nuclear envelope by phosphorylation. Surprise, dephosphorylation
and lamins help to reassemble it.
As you can see, CSEs affect cell shape and sub cell shape. The
plasma membrane is not just dictated by hydrophobic interactions.
Microtubule-Based
Motility
Cilia and flagella must be anchored. The plasma membrane actually
surrounds the flagella. Except for bacterium flagellum, the rest
have a "9+2" arrangement. This includes 9 outer doublets that circle
a central doublet of 2 singlets when looking at the cross section of core
of the cilium or flagellum, called the axoneme. This makes a total
of 20. 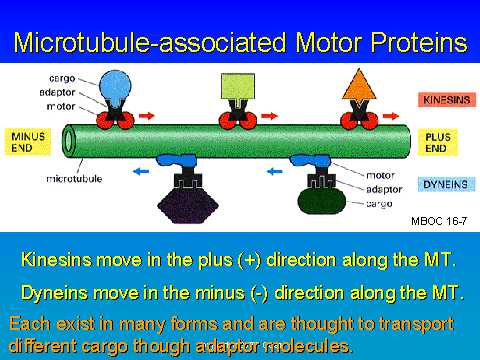 The
circling doublet is made of a complete A tubule and an incomplete B tubule.
A radial spoke extends from the A tubule. The nexin bridge connects
the two types of tubules. The A tubule also has the motor protein,
axonemal (ciliary) dynein. It helps to slide the B tubule to the
negative direction (relative to the A tubule for which it is attached).
The
circling doublet is made of a complete A tubule and an incomplete B tubule.
A radial spoke extends from the A tubule. The nexin bridge connects
the two types of tubules. The A tubule also has the motor protein,
axonemal (ciliary) dynein. It helps to slide the B tubule to the
negative direction (relative to the A tubule for which it is attached).
Flagella movement involves MT and axonemal (ciliary) dynein.
For the nerve cell, the MTOC (-) is located in the cell body and the
MTs extend outward.
Anterograde movement is created by kinesin. It has 2 heads (reversibly
ATP bound) with a hinged stalk. The tail attaches to the cargo (vesicle).
The kinesin receptor on a cell tells it to go anterograde.
Dynein is larger and also has 2 ATPase heads (heavy chain) and a stalk.
In addition, it has light chains. Dyneins act as the retrograde motor
protein.