Cell Biology
Cellular Transport
I think these processes are some of the most
interesting in cell biology so far, but you can judge for yourself.
Membrane
Transport
The cell's main focus is on membrane transport
because they must allow for the movement of molecules into and out of their
bodies. Most animal cells use 1/3 of the energy they produce on the
sodium-potassium pump so I'm sure you are not surprised that cellular transport
is the major activity found in cells. However, not all of these processes
require energy. Diffusion is the movement from high to low concentration
(a.k.a. going with the concentration gradient). Let's take a gander
at the various types of transportation through the plasma membrane.
Simple Diffusion
Simple diffusion is when a solute is allowed
to travel through the lipid bilayer. The rate depends on 1) SOLUBILITY
in the lipid bilayer, 2) CONCENTRATION GRADIENT steepness, and 3) SIZE
(does matter). Usually small inorganic molecules like O2,
CO2, NO, and H20 simply slip past the plasma membrane
between phospholipids. Simple diffusion is non-energy requiring,
but it is also non-selective.
Aquaporins are protein-lined channels that
allow water (polar) to travel through the membrane, which has a nonpolar
middle section. These specialized proteins are made of 6 transmembrane
helix proteins. They function as a homotetramer, and each subunit
has a water channel. There are 10 different types of aquaporins.
Most are constitutive (always expressed), and some are regulated.
An example are the aquaporins in kidney tubules which move from the cytoplasm
into membranes to remove water from urine as a response to vasopressins
Aquaporins are non-energy requiring, but they are selective for water.
Ion channels are transmembrane proteins that
allow ions to move across membranes with their concentration gradient at
high rates. These channels can be control movement by different gating
methods. Ligand gated ion channels allow for transport once a signal
molecule triggers a conformational change. Voltage gated channels
are sensitive to changes of electrical charges in the membrane. Mechanical
forces also contribute to gating. Here's something to think about. 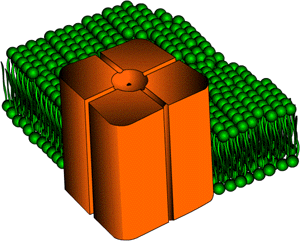 Potassium
ion channels (shown below/left and also in the
background) prefer K+ over Na+ 10,000 to 1.
How do they do this? You can't say the size is perfect for K+
and not Na+ because if you check your periodic table, potassium
is larger than sodium. A channel large enough for the potassium ion
is definitely large enough for the sodium ion so try thinking about how
ions exist in an aqueous solution. Well, they are solvated meaning
that the water molecules hang onto the ions. Both ions do not fit
with waters surrounding them. The dehydration event for K+ is
favored over Na+. The explanation for this is that the
partial negative charge on the oxygens of carbonyl groups of the helices
found along the pore of the ion channel (shown below/right and also
in the background)
will surround the ions like the water molecules once did. The potassium
ion will be chosen over the sodium ion to move through the ion channel
because the carbonyl groups will pack around the K+ in a better,
tighter fashion than around the sodium ion. K+ will be
the one to undergo dehydration and it will now fit into the channel.
Potassium
ion channels (shown below/left and also in the
background) prefer K+ over Na+ 10,000 to 1.
How do they do this? You can't say the size is perfect for K+
and not Na+ because if you check your periodic table, potassium
is larger than sodium. A channel large enough for the potassium ion
is definitely large enough for the sodium ion so try thinking about how
ions exist in an aqueous solution. Well, they are solvated meaning
that the water molecules hang onto the ions. Both ions do not fit
with waters surrounding them. The dehydration event for K+ is
favored over Na+. The explanation for this is that the
partial negative charge on the oxygens of carbonyl groups of the helices
found along the pore of the ion channel (shown below/right and also
in the background)
will surround the ions like the water molecules once did. The potassium
ion will be chosen over the sodium ion to move through the ion channel
because the carbonyl groups will pack around the K+ in a better,
tighter fashion than around the sodium ion. K+ will be
the one to undergo dehydration and it will now fit into the channel. 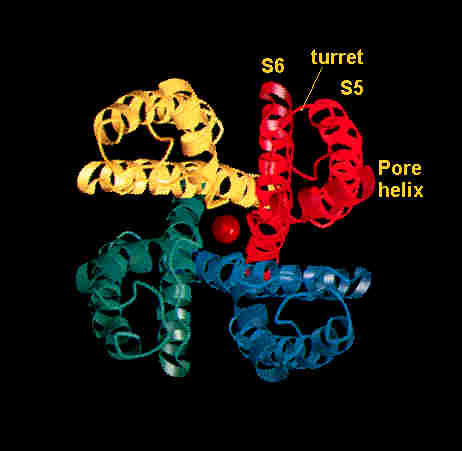 In
this channel, there are at least three binding sites for K+ so
three potassium ions will be in close proximity to each other inside the
protein. Their electrostatic repulsion accounts for the rapid movement
of potassium ions through the ion channel (106 - 109
ions/sec). I must point out that ion channels bring in another variable
with gradients. Not only do you have to consider the concentration
gradient, but you must remember the charge gradient as well. Both
are referred to collectively as the electrochemical or chemical/potential
gradient, which is actually a form of energy. Ions moving through
ion channels are driven by both factors.
In
this channel, there are at least three binding sites for K+ so
three potassium ions will be in close proximity to each other inside the
protein. Their electrostatic repulsion accounts for the rapid movement
of potassium ions through the ion channel (106 - 109
ions/sec). I must point out that ion channels bring in another variable
with gradients. Not only do you have to consider the concentration
gradient, but you must remember the charge gradient as well. Both
are referred to collectively as the electrochemical or chemical/potential
gradient, which is actually a form of energy. Ions moving through
ion channels are driven by both factors.
Ion channels are non-energy requiring, and
they are highly selective.
Facilitated
Diffusion
Now moving on to facilitated
diffusion. This process uses the concentration gradient, but
the twist is that it also receives help from transporters. A great
example is the glucose
transporter. It acts like an enzyme in that its kinetics will
allow it to move a small amount of glucose at a rate proportional to the
concentration, but with the substrate concentration about the KM,
a gradual rate will proceed as it occurs just below VMax.
This transporter is also stereospecific. D-glucose is preferred over
L-glucose. Remember, the glucose transporter is not a channel.
It has binding sites and will change conformation to facilitate diffusion
when glucose binds to it. It refolds to its original shape after
glucose has been released, all of this for a polar glucose molecule to
move through the nonpolar portion of the membrane. Now here's an
interesting biochemical "trick." Once the glucose has been pumped
into the cell, how does the transporter continue to diffuse more of the
sugar in. Will the concentration not build against the inward movement?
Well, the imported glucose is immediately phosphorylated, and technically
it is no longer glucose (it's glucose with a phosphate on it). The
gradient continues to favor input of the sugar because the previous molecules
have been slightly altered, and the phosphorylated glucose is not shipped
out.
Facilitated diffusion is non-energy requiring,
and it is highly selective.
We're not done with glucose transporters just yet. There are plenty
of them. Here are some names:
Glut 1, Glut 2, Glut 3, Glut 4, Glut 5, and
Glut 7 (number 6 turned out to be for fructose - OOPS!)
Some cells will release glucose while others
primarily receive. Either way, these transporters do the job.
The correct transporter is distributed according to the cell's need.
GLUT 2 - found in cells
of organs that release glucose into the blood (liver cells, epithelial
intestine cells). It has a HIGH KM for glucose so its
rate will be proportional to the amount of sugar available.
GLUT 3 - found in the plasma membrane of neuronal
cells of the brain. It has a LOW KM for glucose so its
rate will always have a high affinity for the sugar and keep importing
at a rate near VMax.
GLUT 4 - found in muscle and fat cells.
It is MOBILIZED from its storage granules inside cells and therefore is
responsible for glucose uptake in bursts when it does get to the plasma
membrane.
Active Transport
Active transport is how the cell moves solutes
against their concentration gradients. There is primary active transport
which uses ATP hydrolysis as its energy source. Secondary active
transport uses the electrochemical gradient as its energy source (note
that molecules are still moving against their concentration gradients here
but with the electrochemical gradient). There is also ATP binding
cassette (ABC) transporters.
Let us delve into the primary
active transport examples.
As noted, ATP hydrolysis fuels this transport
against concentration gradient. Ion pumps are involved in the three
classes: P-type, V-type, and F-type
P-type (in plasma membranes and endoplasmic
reticuli): These transporters form a covalently phophorylated (P for phosphorylated)
protein intermediate as part of the transport process. Included in
this class are ion pumps for Ca2+, Na+/K+,
H+, and Na+/H+.
The
well known P class ATPase, the Na+/K+ pump (a.k.a.
Na+/K+ ATPase), consists of a transport protein made
of 2 alpha (for transport by ion pumping) and 2 beta (for regulation) subunits.
The bottom line: for every phosphate used to run the transport protein,
3 Na+ are exported while 2 K+ are brought in. 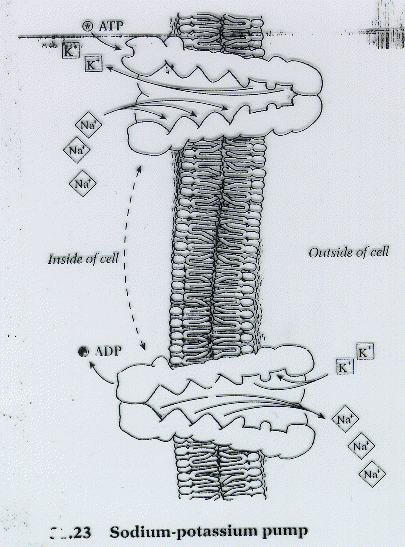 Basically,
the protein (in the E1 conformation) binds 3 Na+,
and hydrolysis of ATP causes a phosphate to induce a conformational change
(to E2) in the transporter. The sodium ions are released
and 2 K+ are bound to the same protein which loses the phosphate
to regain its original E1 conformation so that the potassium
ions can be imported. This mechanism is what creates and maintains
steep concentration gradients like 12 mM of Na+ inside
a cell versus 145 mM of Na+outside the cell and 139 mM of K+inside
a cell versus 4 mM of K+outside the cell.
Basically,
the protein (in the E1 conformation) binds 3 Na+,
and hydrolysis of ATP causes a phosphate to induce a conformational change
(to E2) in the transporter. The sodium ions are released
and 2 K+ are bound to the same protein which loses the phosphate
to regain its original E1 conformation so that the potassium
ions can be imported. This mechanism is what creates and maintains
steep concentration gradients like 12 mM of Na+ inside
a cell versus 145 mM of Na+outside the cell and 139 mM of K+inside
a cell versus 4 mM of K+outside the cell.
The lesser known P class
ATPase, the Ca2+ ATPase, is located in the lumen of the sarcoplasmic
reticulum of muscle cells, in the plasma membrane, and in the membranes
of the endoplasmic reticulum. They release 2 calcium ions from the
cytosol into the sarcoplasmic reticulum, extracellular space, or the lumen
of the ER for every ATP molecule used. The Ca2+ concentration
in the sarcoplasmic reticulum is small. It is smaller in the cytosol.
The KM is the smallest meaning a constant release rate near
VMax.
V-type (in vacuoles, tonoplasts, lysosomes,
endosomes): These pumps use ATP without forming a phsophorylated protein
intermediate. H+ pumps are in this group.
F-type (in bacterial plasma membranes, mitochondral
membranes, chloroplast thylakoid membranes): No phosphorylation here
either, but they do synthesize ATP. (Details are found in the Energy
Transduction section) Once again, H+ pumps are in
this group.
Active transport is energy requiring, and it
is highly selective.
Let us delve into the secondary
active transport examples.
This entails transport against a concentration
gradient coupled to an electrochemical gradient. The principal coupling
ion in animal cells is Na+. How is the Na+
concentration regulated? That's right, the Na+/K+
pump so secondary active transport is actually indirectly coupled w/ ATP
hydrolysis. Now it makes sense that the cell uses 1/3 of its energy
for the Na+/K+ pump.
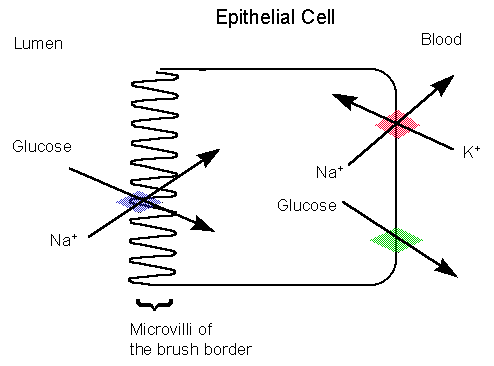 In
the image to the right, glucose enters the apical plamsa membrane of the
epithelial cell of the intestine by secondary active transport. One
glucose molecule and Na+ (actually 2 sodium ions) are carried
inside due to Na+'s concentration gradient (created by
the Na+/K+ pump on the lateral surface which keeps
cytosolic sodium ion concentration low). The glucose molecule comes
along for the ride. This is an example of a symport
(cotransporter) because glucose (against its concentration gradient)
and Na+ (with its gradient) are both moving in the same direction
(into the cell). The movement of 2 sodium ions into the cytoplasm,
which is lesser in sodium ion concentration, changes the conformation of
the transport protein, and glucose is released.
In
the image to the right, glucose enters the apical plamsa membrane of the
epithelial cell of the intestine by secondary active transport. One
glucose molecule and Na+ (actually 2 sodium ions) are carried
inside due to Na+'s concentration gradient (created by
the Na+/K+ pump on the lateral surface which keeps
cytosolic sodium ion concentration low). The glucose molecule comes
along for the ride. This is an example of a symport
(cotransporter) because glucose (against its concentration gradient)
and Na+ (with its gradient) are both moving in the same direction
(into the cell). The movement of 2 sodium ions into the cytoplasm,
which is lesser in sodium ion concentration, changes the conformation of
the transport protein, and glucose is released.
[The coupling of a species moving in the opposite
direction of the entity it is coupled with) describes an
antiport (exchange protein, exchanger).]
The glucose molecules undergo facilitated diffusion
out of the cell and into the blood through the basal lateral membrane by
a GLUT 2 transporter (still remember facilitated
diffusion?).
There are over 200 of the so called ABC
transporters. They have multiple functions (including active
transport of ions), but they all contain common ATP- and nucleotide binding
domains (NBDs). The corresponding polypeptide of the gene responsible
for cystic fibrosis is an ABC transporter which usually functions as a
Cl- channel.
Back
to main page
 Potassium
ion channels (shown below/left and also in the
background) prefer K+ over Na+ 10,000 to 1.
How do they do this? You can't say the size is perfect for K+
and not Na+ because if you check your periodic table, potassium
is larger than sodium. A channel large enough for the potassium ion
is definitely large enough for the sodium ion so try thinking about how
ions exist in an aqueous solution. Well, they are solvated meaning
that the water molecules hang onto the ions. Both ions do not fit
with waters surrounding them. The dehydration event for K+ is
favored over Na+. The explanation for this is that the
partial negative charge on the oxygens of carbonyl groups of the helices
found along the pore of the ion channel (shown below/right and also
in the background)
will surround the ions like the water molecules once did. The potassium
ion will be chosen over the sodium ion to move through the ion channel
because the carbonyl groups will pack around the K+ in a better,
tighter fashion than around the sodium ion. K+ will be
the one to undergo dehydration and it will now fit into the channel.
Potassium
ion channels (shown below/left and also in the
background) prefer K+ over Na+ 10,000 to 1.
How do they do this? You can't say the size is perfect for K+
and not Na+ because if you check your periodic table, potassium
is larger than sodium. A channel large enough for the potassium ion
is definitely large enough for the sodium ion so try thinking about how
ions exist in an aqueous solution. Well, they are solvated meaning
that the water molecules hang onto the ions. Both ions do not fit
with waters surrounding them. The dehydration event for K+ is
favored over Na+. The explanation for this is that the
partial negative charge on the oxygens of carbonyl groups of the helices
found along the pore of the ion channel (shown below/right and also
in the background)
will surround the ions like the water molecules once did. The potassium
ion will be chosen over the sodium ion to move through the ion channel
because the carbonyl groups will pack around the K+ in a better,
tighter fashion than around the sodium ion. K+ will be
the one to undergo dehydration and it will now fit into the channel.  In
this channel, there are at least three binding sites for K+ so
three potassium ions will be in close proximity to each other inside the
protein. Their electrostatic repulsion accounts for the rapid movement
of potassium ions through the ion channel (106 - 109
ions/sec). I must point out that ion channels bring in another variable
with gradients. Not only do you have to consider the concentration
gradient, but you must remember the charge gradient as well. Both
are referred to collectively as the electrochemical or chemical/potential
gradient, which is actually a form of energy. Ions moving through
ion channels are driven by both factors.
In
this channel, there are at least three binding sites for K+ so
three potassium ions will be in close proximity to each other inside the
protein. Their electrostatic repulsion accounts for the rapid movement
of potassium ions through the ion channel (106 - 109
ions/sec). I must point out that ion channels bring in another variable
with gradients. Not only do you have to consider the concentration
gradient, but you must remember the charge gradient as well. Both
are referred to collectively as the electrochemical or chemical/potential
gradient, which is actually a form of energy. Ions moving through
ion channels are driven by both factors.
 Basically,
the protein (in the E1 conformation) binds 3 Na+,
and hydrolysis of ATP causes a phosphate to induce a conformational change
(to E2) in the transporter. The sodium ions are released
and 2 K+ are bound to the same protein which loses the phosphate
to regain its original E1 conformation so that the potassium
ions can be imported. This mechanism is what creates and maintains
steep concentration gradients like 12 mM of Na+ inside
a cell versus 145 mM of Na+outside the cell and 139 mM of K+inside
a cell versus 4 mM of K+outside the cell.
Basically,
the protein (in the E1 conformation) binds 3 Na+,
and hydrolysis of ATP causes a phosphate to induce a conformational change
(to E2) in the transporter. The sodium ions are released
and 2 K+ are bound to the same protein which loses the phosphate
to regain its original E1 conformation so that the potassium
ions can be imported. This mechanism is what creates and maintains
steep concentration gradients like 12 mM of Na+ inside
a cell versus 145 mM of Na+outside the cell and 139 mM of K+inside
a cell versus 4 mM of K+outside the cell.
 In
the image to the right, glucose enters the apical plamsa membrane of the
epithelial cell of the intestine by secondary active transport. One
glucose molecule and Na+ (actually 2 sodium ions) are carried
inside due to Na+'s concentration gradient (created by
the Na+/K+ pump on the lateral surface which keeps
cytosolic sodium ion concentration low). The glucose molecule comes
along for the ride. This is an example of a symport
(cotransporter) because glucose (against its concentration gradient)
and Na+ (with its gradient) are both moving in the same direction
(into the cell). The movement of 2 sodium ions into the cytoplasm,
which is lesser in sodium ion concentration, changes the conformation of
the transport protein, and glucose is released.
In
the image to the right, glucose enters the apical plamsa membrane of the
epithelial cell of the intestine by secondary active transport. One
glucose molecule and Na+ (actually 2 sodium ions) are carried
inside due to Na+'s concentration gradient (created by
the Na+/K+ pump on the lateral surface which keeps
cytosolic sodium ion concentration low). The glucose molecule comes
along for the ride. This is an example of a symport
(cotransporter) because glucose (against its concentration gradient)
and Na+ (with its gradient) are both moving in the same direction
(into the cell). The movement of 2 sodium ions into the cytoplasm,
which is lesser in sodium ion concentration, changes the conformation of
the transport protein, and glucose is released.